In 1931, Dr. Otto Heinrich Warburg was awarded the Nobel Prize in Physiology or Medicine for his discovery that cancerous cells can live and develop in the absence of oxygen at elevated rate of glucose metabolism; a phenotype detected clinically using Positron Emission Tomography (PET). The increase in the glycolytic rate enhances cancer cell proliferation and increases the local concentration of lactic acid, the end product of the glycolytic pathway that creates an unfavorable environment for the normal tissue. Hexokinase, the first enzyme of the glycolytic pathway catalyzes the phosphorylation of glucose to glucose-6-phosphate, is the gateway enzyme of glucose metabolism in the cell. Its irreversible reaction represents a rate-limiting step that is crucial in the regulation of cellular sugar metabolism.
Of the four mammalian isoforms of Hexokinase, isozyme 2 is predominantly over-expressed in different types of cancer especially in those that metastasize and kill their host. Malignant cells chose wisely to over-express Hexokinase 2 (HK2), the isoform with the greatest catalytic potential and glucose binding affinity. HK2 is the only mammalian isoform with catalytically active N- and C-terminal domains, and it is strategically positioned on the outer mitochondrial membrane (OMM) through interactions by its hydrophobic N-terminal helix to promote interactions with the voltage-dependent anion channel (VDAC) that facilitate the diffusion of ATP from the mitochondria. HK2 interaction with VDAC does not only gives it preferential access to ATP that is much needed for its enzymatic activity but more importantly it inhibits apoptosis in cancerous cells. HK2 interaction with VDAC prevents the release of cytochrome c or association with proapoptotic factors (Bad and Bax) to render the formation of a mitochondrial permeability transition pore that otherwise leads to mitochondrial swelling and cell death. As a result, HK2 regulation can be used to control the rate of glycolysis and cellular growth in tumor cells and its interactions with the OMM prevents apoptosis and increases cancer longevity, a model that we use to characterize the role of HK2 in cancer initiation and maintenance and to screen and characterize new class of anticancer therapeutics that inhibit HK2 enzymatic function and localization to the OMM.
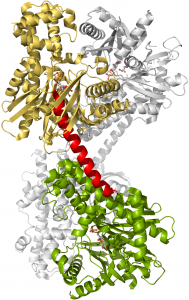
Crystal Structure of Human Hexokinase 2
The crystal structure of HK2 (PDB code: 2NZT) was solved in complex with Glucose and Glucose 6-Phosphate. The enzyme is a homodimer with monomers containing N- (gold) and C-terminal (green) domains that are identical and possess active site for the phosphorylation of glucose to glucose 6-phosphate. The two domains are linked by a long eight turn α-helix (red) with each catalytic domain contains small and large sub-domains that sandwich the active site. The two sub-domains are connected by a small 3 turn α-helix that functions as a hinge to open and close the active site for the binding of substrates and release of products.
![]()